- 29 Jan, 2021
- /
- Category:
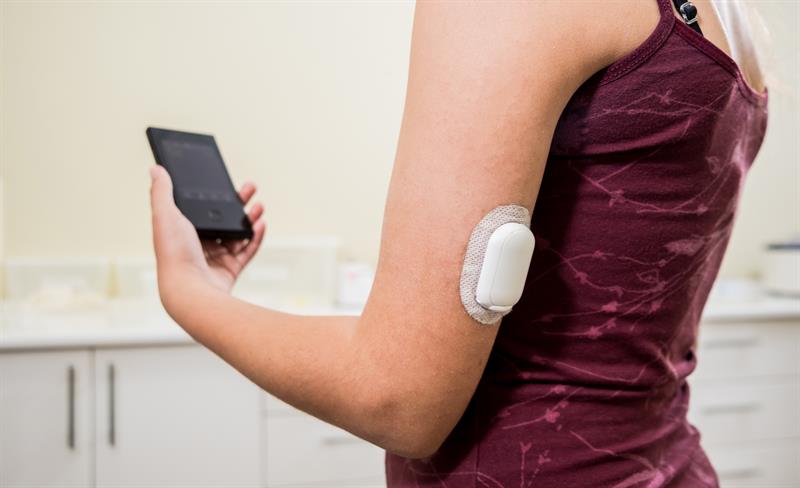
Testing Wearable Medical Device Adhesion During Development Stage
The wearable adhesives market is growing rapidly, driven by provider and consumer demand as well as advancements in medical devices for personalized care such as continuous glucose monitoring and point-of-care diagnostics. Wearable adhesives are used to attach medical devices to the body for continuous and long-term durations; the ability to properly adhere to the skin is a key factor for an effectively functioning device.
The selection and development of this adhesive is challenging and needs careful consideration due to the complex and varying nature of human skin and the characteristics of the specific wearable device, such as its size, weight, flexibility, and application purpose. While proper screening and testing to choose the optimal wearable adhesive may seem time and labor intensive, it can ultimately save time and money by reducing risk in the long run. Moving forward with an existing adhesive carrier substrate without careful evidence might lead to failure at the clinical stage because of improper device adhesion.
In order to identify the adhesive that is the best choice for a particular wearable device, Scapa Healthcare first defines the requirements for the device and its adhesive, including:
- Targeted users
- Application site
- Wear duration for individual application and number of expected re-applications
- Condition of use (at rest or active patient)
- Size and weight of the device
- How the device will be attached to the adhesive patch
- Whether the device will cause some occlusion of the patch or allow sweat to pass though the patch
Prototype Screening
To save time during development, the screening process of the possible solutions is fundamental. An effective combination of a few in vitro and in vivo tests can drastically accelerate the development cycle. Base characteristics of the adhesive are moisture vapor transmission rate (MVTR), peel, tack and shear. All of those properties can be effectively screened in vitro. Scapa compares adhesive options to one another in terms of tack, peel, and MVTR which will drive informed decisions on the adhesive selection and will facilitate a new selection in the event the first choice of adhesive does not perform as expected in a healthy volunteer study.
Selection Process
The next step is to classify the adhesive prototypes from the highest to the lowest tack, peel, and MVTR. Next, the appropriate carrier will need to be selected using flexibility, durability, weldability, and breathability requirements.
Volunteer Study
Based on the classification of the adhesive, we conduct a study on healthy volunteers with the wearable device itself (or a surrogate that has similar weight, shape and thickness) at the application site. During human testing, biocompatibility factors such as cytotoxicity, skin irritation and sensitization are evaluated.
How Scapa Healthcare’s team can support your next innovation
Scapa Healthcare is a trusted, strategic partner of choice for contract manufacturing in the development of custom medical-grade skin solutions for medical device fixation. Scapa delivers expert support and innovative technologies from concept to development to full-scale production.
Learn more about Scapa Healthcare’s adhesives for wearable medical device fixation here.
